RESEARCH INTERESTS
1. 4D (3D spatial + 1D temporal) meiotic genomes in spermatocytes and oocytes
Chromosome conformation and organization impact many cell functions, including transcription, homologous chromosome pairing, and chromosome segregation. Understanding the chromosome architecture and dynamics is critical for developing effective strategies to cure human diseases, such as cancer, miscarriage, infertility, and birth defects. Our vision is to establish a new paradigm and open up new avenues of investigation into the poorly understood physical and conformational aspects of mitotic and meiotic chromosomes. Globally, individualized and compacted chromosomes provide a structural basis for the pairing and segregation of homologous chromosomes. Locally, chromatin organizations, topological associating domains (TADs) and pair-wise-chromatin-loop interactions, regulate meiotic transcription. Another larger chromatin domain, compartment, is associated with meiotic recombination. It remains unknown how the conformational changes of meiotic chromosomes impact meiotic chromosome pairing, recombination, segregation, and transcription. This project aims to understand: 1. how meiotic chromatin fibers fold into rod-like chromosomes; 2. how these dynamic conformational changes regulate transcription, facilitate homologous chromosome pairing and segregation.
We utilized four approaches to investigate meiotic chromosomes:
-
Computational approaches – chromosome conformation capture (Hi-C)
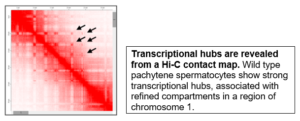
-
Biophysical approaches – micromanipulation
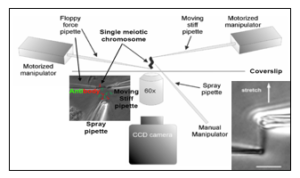

Ronald Biggs, Ning Liu, Yiheng Peng, John F. Marko^, Huanyu Qiao. Micromanipulation of prophase I chromosomes from mouse spermatocytes reveals high stiffness and gel-like chromatin organization. Communication Biology.
-
Cytological approaches – super-resolution microscopy
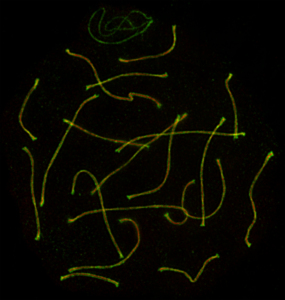

-
MINimal fluorescence photon FLUXes (MINFLUX)
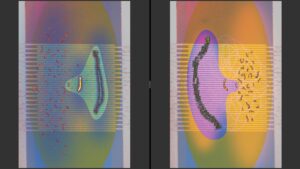

2. The roles of SUMOylation in meiotic checkpoint pathways
Oocyte quality control cull eggs with defects in meiosis for apoptosis and determines the size of perinatal oocyte pool and further reproductive lifespan. In mice, postnatal oocyte death is triggered by defects during synapsis and recombination that involve repairing programmed DNA double-strand breaks (DSBs) between homologous chromosomes.
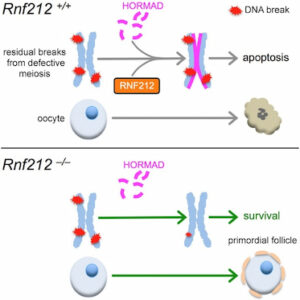
We show that RNF212, a SUMO E3-ligase, functions in oocyte quality control. Both physiological apoptosis and the wholesale elimination of oocyte pool in meiotic mutants require RNF212. Our data indicates that RNF212 impedes DNA DSB repair when homologous chromosomes separate by blocking recombination between sister chromatids. Consistently, HORMAD1, a negative regulator of inter-sister recombination, requires RNF212 for its association with separating chromosomes. We infer that by blocking the repair of residual DNA DSBs, oocytes retain a “memory” of defective inter-homolog interactions that enable quality control processes. Together, these results define the logic of postnatal oocyte quality control and suggest that RNF212 variants may influence the transmission of defective genomes.
The long-term goal of this research project is to understand how post-translational modifications, such as SUMOylation (Small Ubiquitin-like Modifier) and ubiquitination, regulate a range of checkpoint pathways in mammalian cells. We will utilize many experimental methods and techniques, such as single-cell RNAseq, mouse genetics, proteomics, electron microscopy, super-resolution microscopy, and spermatocyte/oocyte culture.
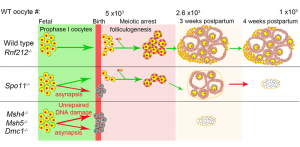
This work has the potential to uncover fundamental molecular mechanisms that will provide insights for developing treatments for infertility, miscarriage, birth defects, cancer, neural degeneration, and aging-related diseases.
Huanyu Qiao, H.B.D. Prasada Rao, Yan Yun, Sumit Sandhu, Jared H. Fong, Manali Sapre, Michael Nguyen, Addy Tham, Benjamin W. Van, Tiffany Y.H. Chng, Amy Lee, Neil Hunter. (2018). Impeding DNA Break Repair Enables Oocyte Quality Control. Molecular Cell, 72, 211-221. PMID: 30270110
3. The effects of environmental toxicants on meiosis
Meiosis is crucial for sexual reproduction. Many environmental toxicants, such as endocrine disrupting chemicals (EDCs), disinfection by products (DBPs), and per- and polyfluoroalkyl substances (PFAS), disrupt meiosis and induce infertility, miscarriage, and chromosomal birth defects. These disruptions could affect several generations in a multigenerational and/or transgenerational manner. Unfortunately, it is impossible for us to remove these chemicals from our environment in the near future due to their ubiquity and stability. Understanding the molecular mechanisms of how these factors exert toxic effects is critical for developing effective therapeutic strategies to prevent the aforementioned harmful outcomes. In addition, our studies can help government agencies such as the U.S Environmental Protection Agency set legal limits on these chemicals. Using in vitro prenatal ovary and oocyte culture, we are able to investigate the effects of various environmental toxicants on the meiosis and oocyte maturation.
We have demonstrated that two environmental factors, phthalate and a high-fat diet, can synergistically impact oogenesis and folliculogenesis. The oocytes from F1 fetal mice experienced elevated abnormal gene silencing in certain chromosomal regions after the pregnant F0 dam was treated with both a high-fat diet and di-(2-ethylhexyl) phthalate (DEHP). After birth, the development of preantral follicles was also affected by the DEHP-and-high-fat-diet combined treatment. Our data suggests that pregnant women could carefully choose their diet to avoid adverse multigenerational effects caused by DEHP.

Supipi Mirihagalle, Tianming You, Lois Suh, Chintan Patel, Liying Gao, Saniya Rattan, Huanyu Qiao. (2019). Prenatal exposure to di-(2-ethylhexyl) phthalate and high-fat diet synergistically disrupts mouse fetal oogenesis and affects folliculogenesis. Biology of Reproduction, 6, 1561-1570. PMID: 30939196
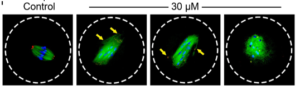

We also studied another environmental pollutant, iodoacetic acid (IAA). IAA is a byproduct of the disinfection process of our drinking water. We found that IAA adversely affected oocyte maturation process by elevating ROS levels, disrupting spindle assembly, inducing DNA damage, and causing MI arrest, which poses risks to female reproductive health. We also showed how an antioxidant, N-acetyl-L-cysteine (NAC), could rescue IAA-induced abnormalities in mouse oocytes, indicating that oxidative stress is a major cause of oocyte maturation failure after IAA exposure.
Xiaofei Jiao, Andressa Gonsioroski, Jodi A Flaws, Huanyu Qiao. Iodoacetic acid disrupts mouse oocyte maturation by inducing oxidative stress and spindle abnormalities. Environmental Pollution.
 PFAS are man-made chemicals that are widely used in various products. However, it is still unclear how the structures of PFAS, such as carbon-chain length and functional groups, determine their reproductive toxicity. Our study provides new information on the structure-toxicity relationship of PFAS. Specifically, at 600 μM, perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS) reduced the rates of both germinal-vesicle breakdown (GVBD) and polar-body extrusion (PBE), enlarged polar bodies and induced excess reactive oxygen species (ROS) and decreased mitochondrial membrane potential (MMP). These meiotic defects compromised oocyte developmental competence after parthenogenetic activation.
PFAS are man-made chemicals that are widely used in various products. However, it is still unclear how the structures of PFAS, such as carbon-chain length and functional groups, determine their reproductive toxicity. Our study provides new information on the structure-toxicity relationship of PFAS. Specifically, at 600 μM, perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS) reduced the rates of both germinal-vesicle breakdown (GVBD) and polar-body extrusion (PBE), enlarged polar bodies and induced excess reactive oxygen species (ROS) and decreased mitochondrial membrane potential (MMP). These meiotic defects compromised oocyte developmental competence after parthenogenetic activation.

Jianan Feng, Edgar J. Soto-Moreno, Aashna Prakash, Ahmed Z. Balboula, Huanyu Qiao. Adverse PFAS effects on mouse oocyte in vitro maturation are associated with carbon-chain length and inclusion of a sulfonate group, Cell Proliferation
4. Analysis of meiotic silencing by “digital-chromosome-banding”
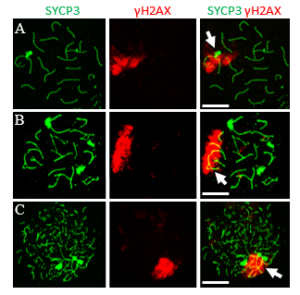
To safeguard meiotic progression, evolutionarily conserved checkpoint mechanisms monitor the fidelity of homologous synapsis and recombination. One key mechanism involved is meiotic silencing of unsynapsed chromatin (MSUC), which is initiated by the DNA damage response (DDR) pathway, as marked by γH2AX. In male mammals, this process is normally restricted to the unsynapsed sex chromosomes, a specialized form known as meiotic sex chromosome inactivation (MSCI), which is required for successful spermatogenesis. Although the initiation of MSCI has been well characterized, its full silencing dynamics and the structural mechanisms that sustain it remain incompletely understood. In contrast to MSCI, broader MSUC can occur on autosomes in response to synapsis failure, but its cell-to-cell variability has made it difficult to quantify and compare.
 To overcome this limitation, we developed “digital-chromosome-banding,” a single-cell-based approach that enables quantitative, region-specific analysis of transcriptional repression, allowing us to systematically dissect the spatial and temporal dynamics of MSCI and MSUC.
To overcome this limitation, we developed “digital-chromosome-banding,” a single-cell-based approach that enables quantitative, region-specific analysis of transcriptional repression, allowing us to systematically dissect the spatial and temporal dynamics of MSCI and MSUC.

5. Measurement of spindle and chromosome stiffness via micromanipulation
Chromosome structure is complex, and many aspects of chromosome organization are still not understood. Measuring the stiffness of chromosomes offers valuable insight into their structural properties.
 In our study, we identify differences in stiffness between meiosis I and II chromosomes, as well as an age-dependent increase in stiffness in meiosis I and meiosis II chromosomes. We also examine the role of meiosis-specific cohesin complexes in regulating chromosome stiffness. Since aging is associated with elevated levels of DNA damage, we investigated the impact of etoposide-induced DNA damage on chromosome stiffness and found that it led to a reduction in stiffness in MI oocytes. Our study underscores the dynamic and cyclical nature of chromosome stiffness, modulated by both the cell cycle and age-related factors.
In our study, we identify differences in stiffness between meiosis I and II chromosomes, as well as an age-dependent increase in stiffness in meiosis I and meiosis II chromosomes. We also examine the role of meiosis-specific cohesin complexes in regulating chromosome stiffness. Since aging is associated with elevated levels of DNA damage, we investigated the impact of etoposide-induced DNA damage on chromosome stiffness and found that it led to a reduction in stiffness in MI oocytes. Our study underscores the dynamic and cyclical nature of chromosome stiffness, modulated by both the cell cycle and age-related factors.
Ning Liu, Wenan Qiang, Philip Jordan, John F Marko, Huanyu Qiao. Cell-cycle and Age-Related Modulations in Mouse Chromosome Stiffness. Elife.
Spindles are essential for accurate chromosome segregation in all eukaryotic cells.
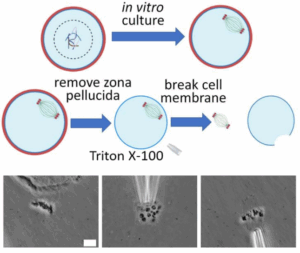 To enable detailed investigation of their physical properties and functions, we describe an innovative method for isolating fresh, intact spindles from mouse oocytes. Using this method, we were able to isolate and analyze spindles at different meiotic stages. We found that the spindle length decreases upon isolation from the oocyte. Combining this observation with direct measurements of spindle mechanics, we examined the forces governing spindle migration during oocyte asymmetric division. Our findings suggest that the spindle migration is regulated by a pulling force and a net tensile force of approximately 680 pN is applied to the spindle in vivo during the migration process.
To enable detailed investigation of their physical properties and functions, we describe an innovative method for isolating fresh, intact spindles from mouse oocytes. Using this method, we were able to isolate and analyze spindles at different meiotic stages. We found that the spindle length decreases upon isolation from the oocyte. Combining this observation with direct measurements of spindle mechanics, we examined the forces governing spindle migration during oocyte asymmetric division. Our findings suggest that the spindle migration is regulated by a pulling force and a net tensile force of approximately 680 pN is applied to the spindle in vivo during the migration process.

Ning Liu, Ryo Kawamura, Wenan Qiang, Ahmed Balboula, John F Marko*, Huanyu Qiao* . Isolation and manipulation of meiotic spindles from mouse oocytes reveals migration regulated by pulling force during asymmetric division. BioRxiv
Interview with 2022 SSR Janice Bahr Junior Scientist Travel Awardee Dr. Joe (Huanyu) Qiao
https://www.buzzsprout.com/1786803?client_source=large_player&iframe=true&referrer=https://www.buzzsprout.com/1786803.js?container_id=buzzsprout-large-player&player=large#
SSR interview Profile of Dr. Qiao: https://ssr.org/news-events/ssr-reflects/ssr-diversity-dr-huany-qiao.html
Interview with Cancer Center at illinois
https://cancer.illinois.edu/people/huanyu-qiao/
CCIL seed funding: https://cancer.illinois.edu/groundbreaking-dna-repair-research-receives-cancer-center-at-illinois-seed-grant/
