RESEARCH PROJECTS
Our lab uses animal models to understand how neuromodulators contribute to sensory and memory encoding and how this can facilitate interactions across regions of the brain. We utilize optogenetic and chemogenetic targeting tools to measure how perturbations of neurotransmitter systems associated with illness or disease alter sensory encoding and the coupling of neurons and brain regions together to ultimately influence learning and memory or cognitive and social behavior in mice.
1. OSCILLATIONS IN CIRCUIT FUNCTION
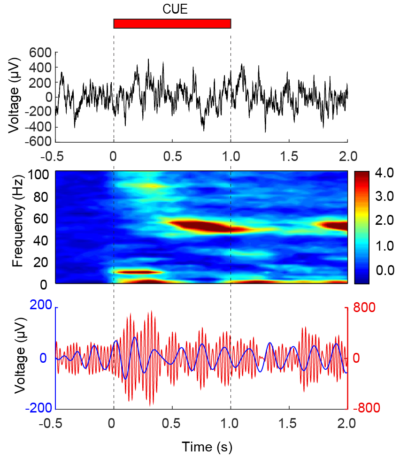
How do distinct regions of the brain interact as a process of what we experience and how does that information modulate perception? It is a mystery as to how coordination between brain regions occurs, but overwhelming evidence suggests that rhythms or “brain waves” that are present in all animal species serve as a type of scaffolding that allow for the dynamic coupling of regions together transiently. In diseases such as autism, we believe that local rhythms contribute so strongly to couple nearby neurons that they are not influenced by other regions of the brain. In contrast, in conditions like Parkinson’s disease, we believe that regions of the motor pathways become so ubiquitously over-coupled that it impedes the ability to promote movement. We aim to understand the mechanisms important for the generation and timing of rhythms that temporally couple neurons and how this process aids cognitive performance and how changes in normal rhythms can contribute to neuropsychiatric illness and disease.
2. PERCEPTUAL ENCODING IN COMPLEX AUDITORY SCENES

A cocktail party, filled with sounds coming from multiple directions, exemplifies how acoustic stimuli compete for our attention. The biological auditory system has evolved to manage this complexity effectively. However, the specific mechanisms by which the auditory cortex successfully suppresses competing stimuli so that only the attended stream is represented remains largely unknown. One of the projects in the labs aims to uncover the mechanisms of attention and cortical inhibition that help isolate specific sound streams from background noise within the auditory cortex. To address this challenge, we employ a combination of cutting-edge techniques. These include novel imaging and electrophysiology methods to observe the neural activity in the auditory cortex in vivo, optogenetics to selectively control the activity of different neuron types, and machine learning algorithms designed to assess how neurons differentiate between spatial sounds. Through this multifaceted approach, we hope to provide new insights into the neural basis of auditory attention, inhibition, and spatial encoding.
3. SENSORY PROCESSING IN ANIMAL MODELS OF AUTISM
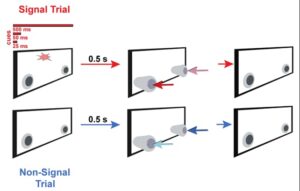

One of the most common phenotypes present in individuals with autism includes sensory hypersensitivity and difficulty hearing in complex sensory environments. Genetic studies have revealed several genetic mutations that are highly correlated with ASD. In our lab we use a mouse model of Fragile X Syndrome, or the FRM1-knockout mouse line to study sensory encoding in complex environments. We utilize various imaging techniques including calcium imaging, fiber photometry and optogenetics to dissect the neural circuitry recruited in auditory processing and how it differs in the mouse model of ASD. Currently we are investigating how the cholinergic system could potentially play a role in the auditory processing impairments noted by ASD patients.
4. HOMEOSTATIC REGULATION OF NEURAL CIRCUITS

How is information processing regulated with neural circuits and how does imbalance in neuromodulators and excitation/inhibition contribute to neuropsychiatric illness? Imbalances between excitation and inhibition (E/I) represent a common pathological feature in neurodevelopmental and neurodegenerative disorders such as Parkinson’s Disease and Autism. A variety of genetically distinct disease models commonly share imbalanced synaptic conductance ratios and abnormal brain oscillations. Despite the shared prevalence of E/I imbalance, abnormal rhythms, and the presence of motor, social, or cognitive deficits, there is no underlying unifying theory to connect these features. Our lab is interested in identifying the mechanistic foundation of imbalance and how does imbalance contribute to cognitive or behavioral deficits using high density recordings from known cell types across aging and disease. By utilizing virtual reality, high-resolution imaging, and behavioral analysis in transgenic models, we aim to uncover how these neural interactions contribute to motor symptoms and identify potential therapeutic targets for neuromodulatory imbalance.
5. ENVIRONMENTAL RISK FACTORS FOR NEURODEGENERATIVE AND NEURODEVELOPMENTAL DISORDERS

Our lab utilizes comprehensive approaches including whole-brain network imaging using functional and structural MRI and multiregional real-time neurotransmitter signaling during behavior to understand how endocrine disrupting chemicals (EDC’s) might contribute to the etiology of neurodegenerative and neurodevelopmental disorders including Alzheimer’s Disease (AD) and autism spectrum disorder (ASD). Humans are exposed to a variety of environmental chemicals including plasticizers in daily life through ingestion, inhalation and dermal contact. One of these chemicals is phthalates which are known endocrine disruptors, and the risk of increased susceptibility to developing certain neurodegenerative diseases through exposure is currently unknown. Overall our goal is to understand how this early-life exposure might contribute to the incidence of AD and ASD phenotypes later in life.
